Sanitation
At Children’s Dental Centre, we take the safety of our staff and our patients very seriously. As aligned with the CDC and ADA, we follow strict sanitation guidelines. In our office, we believe you can never be ‘too safe or too clean’! That’s why we’ve partnered with the professionals at MediClean & Shield (MCS) for the ultimate infection prevention and surface disinfecting practice. The MCS team uses a patented and EPA approved surface disinfecting and treatment application, along with a set of proprietary surface testing and water testing protocols that measure microbial contamination levels for proven, lasting results!
MCS offers technology enhanced infection control for all types of health care facilities. Read below to see who they are and what they do to help us keep our facility virus free and safe!
Infection Control Guidelines for Dental Offices
To better understand infection prevention, let’s start by reviewing the Centers for Disease Control’s list of “Standard Precautions”, which are the minimum infection prevention practices that apply to all types of patient care. Standard Precautions in dental and orthodontic offices include:
- hand hygiene
- use of personal protective equipment (PPE) like masks, gloves, and eye protection
- clean and disinfected surfaces
- sterile instruments and devices
- respiratory hygiene/cough etiquette
- sharps safety (engineering and work practice controls)
- safe injection practices
The CDC admits that these Standard Precautions alone cannot prevent all types of transmissions. A second tier of infection prevention is needed when patients have diseases that can spread through contact, droplet or airborne routes (e.g., skin contact, sneezing, coughing). However, dental offices are not typically required to carry out all of the Transmission-Based Precautions that are recommended for hospital and other ambulatory care settings, because patients usually don’t seek routine dental care when they’re ill with these types of diseases.
With coronavirus variants still circulating, the CDC continues to update its guidance to protect both dental health care professionals, their patients, and dental office visitors.
Read more about CDC guidelines for dental practices >
Unlike CDC recommendations, which are advisory in nature, the US Department of Labor Occupational Safety and Health Administration’s (OSHA) guidance contains references to mandatory requirements under OSHA standards. To help prevent the spread of COVID-19, OSHA recommends measures such as working from home or flexible work schedules, increased ventilation in the work environment, encouraging vaccinations, wearing PPE and face coverings, physical distancing, and enhanced cleaning programs with a focus on high-touch surfaces.
Read more about OSHA guidelines relating to COVID and the work environment >
Dental Unit Waterline (DUWL) Maintenance and Testing
In addition to airborne virus prevention and surface disinfection, another key component in a dental/orthodontic practice is routine maintenance of dental unit waterlines. Because waterline management systems can vary, every office should have a waterline protocol, and be trained on waterline maintenance procedures specifically tailored to their office’s dental unit. So before conducting any waterline maintenance, contact the manufacturer for specific instructions on how to best disinfect the system without causing damage to it.
Why is it important to maintain specific levels of water quality?
Studies have shown that DUWLs can become colonized with microorganisms (like bacteria, fungi, and protozoa), which can colonize and replicate on the interior surfaces of the waterline tubing, often forming a biofilm that amplifies the number of free-floating microorganisms in water used for routine dental procedures.
The presence of substantial numbers of pathogens in dental unit waterlines has generated a growing concern about procedural water quality. In 1993 the CDC recommended that DUWLs be flushed every morning to reduce the microbial build-up. However, follow-up studies indicated this practice did not affect the biofilm in the waterlines or reliably improve the water quality. In 1995 the ADA began asking DUWL manufacturers to provide equipment with the ability to deliver treatment water with <200 CFU/ml of unfiltered output from waterlines.
The majority of organisms recovered from dental waterlines are common heterotrophic water bacteria, but in large numbers these can have a negative impact on patients.
Read more about CDC Guidelines for infection control in DUWLs >
Disinfecting Protocols for DUWLs
The Environmental Protection Agency, together with the American Public Health Association, and the American Water Works Association, have set regulatory standards for safe drinking water, limiting the number of heterotrophic bacteria in water to a minimum of <500 CFU/ml. To achieve water quality of 500 CFU/ml or less, it is important to note that dental/orthodontic offices should implement a self-contained water supply system as part of their quality control strategy.
The Organization for Safety, Asepsis and Prevention (OSAP) recommends monitoring and testing DUWLs every 3 months to ensure waterlines contain <500 CFU/ml.
In most cases, following these protocols will ensure compliance with waterline recommendations:
- Use an approved waterline disinfectant solution. At minimum, this should be run through the dental unit once a week or use a full-time disinfectant option.
- Purge the dental unit with air at the end of each week and let dry. To do this, remove and empty the water bottle then reinstall it. Remove the handpicks. Hold the handpick tubing and air/water syringe over a sink or bucket and run until all water is purged from the system. Turn the unit off and allow to dry over the weekend.
- It is also advisable to use an approved biofilm remover to “shock” the system at least quarterly.
- Regular water analysis reports can aide in risk management! Test dental water units quarterly to make sure bacterial levels remain less than 500 CFU/ml of water.

Read more about MCS waterline microbial testing >
Effective Disinfectants against Viruses
After living through a pandemic, we all know that disinfecting and routinely cleaning commonly touched surfaces helps to kill viruses, but do all disinfectant’s work the same on all viruses?
Let’s start with the basics on viruses… they’re a microscopic parasite, meaning they do not contain components of normal living organisms like plants, animals or bacteria because they cannot reproduce without a host. To reproduce they attach and use their genetic material to trick the host cell into making more copies of the virus. The catch is, they must enter the proper host’s cells. For example, because SARS-CoV-2 is a respiratory virus, it primarily reproduces within cells that line the respiratory tract.
Regardless of their host, viruses can be divided into different categories based on their genome structure, shape (morphology), size, and presence or absence of a lipid membrane called an envelope. Depending upon the virus type and environmental conditions, viruses can survive for extended periods on surfaces; remaining infectious even after several days.
The COVID-19 Coronavirus (named after its ‘crown’ shape when viewed under an electron microscope) is an “enveloped RNA virus”. All coronaviruses have a similar structure and can stick to surfaces but are also able to be killed with disinfectants. Therefore, cleaning, sanitizing and disinfecting surfaces is a key component in the ongoing fight to eliminate these microbial threats!
PermaSafe™ is an EPA Approved Hospital-Grade Disinfectant
The PermaSafe™ system is a patented and EPA-registered disinfection & long-term microbial control system and surface protectant for virtually every common surface or substrate within any environment.
PermaSafe’s products have been approved by the EPA for use against SARS-COV-2, the coronavirus that causes COVID-19. (Please note, only the EPA regulates claims on disinfectant product labels. Before a company can sell or market a disinfectant whose label claims to kill a certain pathogen, the EPA must authorize that claim during the registration process. All claims must be supported by sound science and testing data.) PermaSafe CLEAN meets the EPA’s emerging pathogen requirements for viruses showing efficacy against a wide range of enveloped and non-enveloped viruses, both large and small.
When following product label directions, the EPA specifies that disinfectant products on List N are approved to kill SARS-CoV-2, the coronavirus that causes COVID-19, because they:
- Demonstrate efficacy against the coronavirus SARS-CoV-2 (COVID-19);
- Demonstrate efficacy against a pathogen that is harder to kill than SARS-CoV-2 (COVID-19); or
- Demonstrate efficacy against a different human coronavirus similar to SARS-CoV-2 (COVID-19).
The Science behind PermaSAFE
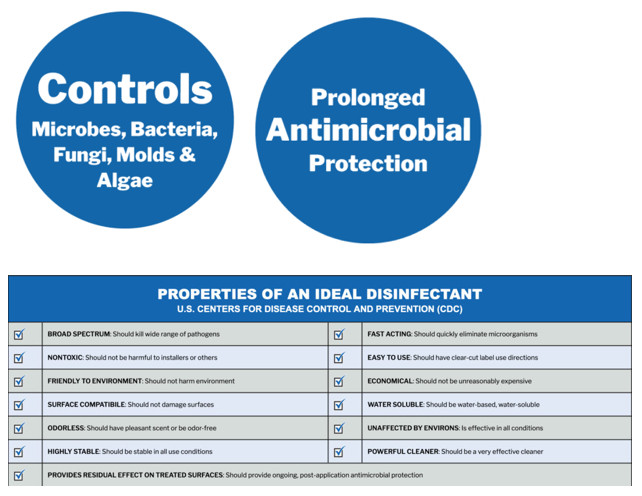
PermaSafe is a patented and EPA registered, broad-spectrum, hospital-grade disinfectant that eliminates up to 99.999% of bacteria, viruses, fungi, mold, mildew and other harmful microbes that can be hazardous to a person’s health.
Effective antimicrobials are not new to the market, however the PermaSafe system provides continuous antimicrobial control for up to 3 months without the repeated use of harmful, toxic chemicals that conventional disinfectants and antimicrobials use.
We also love PermaSafe because it’s odor free, skin contact safe, non-toxic, non-flammable, non-corrosive, non-hazardous; safe to use on electronics; environmentally neutral and eco-friendly!
How do you know if a surface is REALLY clean? You TEST it!
“This product gets under and around hard-to-reach surfaces that routine wiping sometimes misses. The quarterly test results prove our office surfaces remain clean and safe for 3 months… and without using any harsh chemicals!” – Dr. Greg Greenberg
Here's how the Process Works
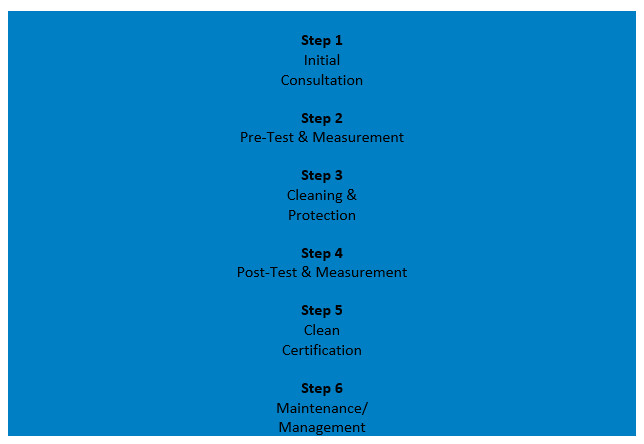
MCS has years of experience in the health care industry and is an industry leader in infection control techniques and cutting-edge technology. Anyone can clean an office with disinfectant sprays, but MCS backs their results with rapid microbial and ATP testing data that proves that Children’s Dental Centre is more than the standard ‘clean’! Their proprietary 6 step process not only checks, but confirms, that all our office environment surfaces remain anti-microbial and anti-viral for up to 3 months between cleaning and protecting spray treatments. This gives us peace of mind that everyone who enters and leaves our office is safe!
MCS uses the PermaSafe system, a 2-step disinfection and protection process using two key products: PermaSafe CLEAN (Step 1) and PermaSafe SHIELD (Step 2) applied with an electrostatic sprayer.
PermaSafe CLEAN is the patented multi-purpose, multi-surface, broad-spectrum, germicidal cleaner, food contact surface sanitizer, and hospital disinfectant that deep cleans and decontaminates surfaces to ensure the subsequent application of PermaSafe SHIELD achieves an effective, durable protective bond. It’s also EPA and NSF Registered, FDA Approved and CDC Compliant.
PermaSafe SHIELD is the patented antimicrobial agent that molecularly bonds to the surface to which it is applied, effectively becoming a permanent part of that surface. As the coating dries, millions of microscopic antimicrobial “spike-like” appliances form and extend from the surface. These “spikes” are actually long ISO polymer chains of positively charged atoms that represent a deadly dual threat to microbes.
Once cured, the antimicrobial coating forms an invisible protective shield that inhibits the growth of microorganisms and helps prevent the growth and spread of bacteria. Because the cell walls of harmful microorganisms are negatively charged, they are electrostatically drawn towards and pulled onto the positively charged spikes and destroyed, as the spikes both impale and electrostatically “shock” the microbes.
The electrostatic sprayer method kills up to 99.999% of pathogens. It also kills the COVID-19 virus.
Proven Test Results
MCS routinely swabs and tests our office for both ATP and microbial activity:
- ATP (Adenosine Triphosphate) is an energy molecule found in all plant, animal and microbial cells… basically the food for microorganisms to grow.
- Microbial activity refers to microorganisms such as viruses, bacteria, and fungi that can infect humans and pets. Since these microorganisms need a food source to live and grow, they feed off of ATP.
Spot testing surfaces ensures that our office remains free of these harmful microorganisms, and that our office environment won’t be a breeding ground for future microbial growth.
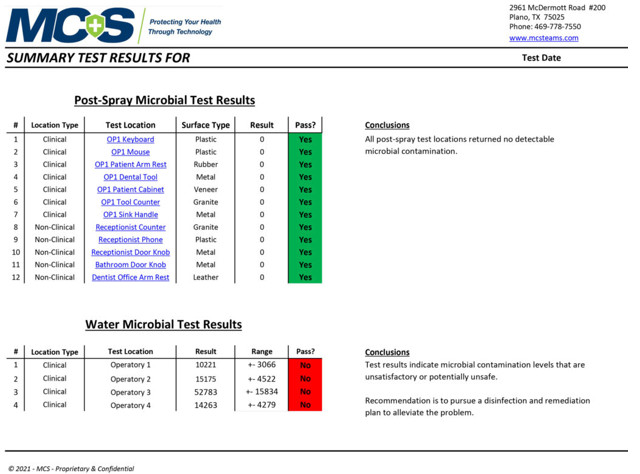
Sample Summary Test Results
An MCS Infection Prevention Specialist will conduct multiple tests in key locations to determine the amount of active organic matter through industry-standard ATP and/or microbial testing procedures. The pre-spraying results will be documented and recorded as a baseline comparison. After the 2-step disinfection and protection treatment process is complete, a post-spraying test will be conducted on the same surfaces to verify the results of the cleaning/disinfecting process. (Below are sample pre- and post-test results provided by MCS to show data measurements. They do not reflect results specifically taken from our office.)
Read more about electrostatic spraying and microbial testing >
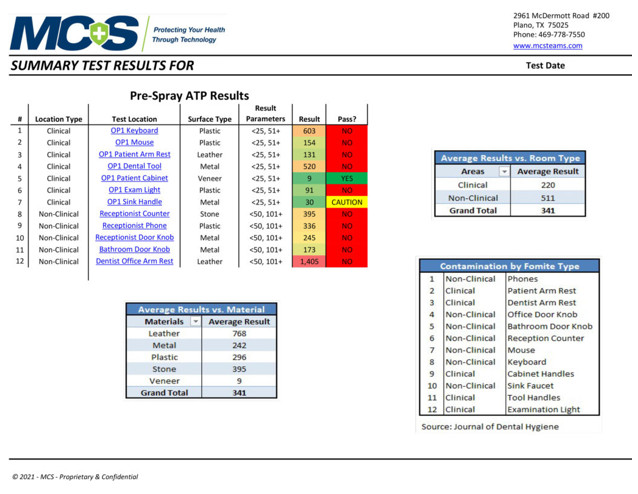
Pre-Spray ATP Test Results
Post-Spray Microbial Test Results & Water Test Levels
Got Questions?
We encourage you to dive deeper and learn more about the MCS team and PermaSafe’s infection control products we use to keep our office environment, staff and patients protected against germs and infections. Learn about MCS infection control services > or Read more about the science behind electrostatic spraying and microbial testing >